A - B - C - D - E - F - G - H - I - J - K - L - M - N - O - P - Q - R - S - T - U - V - W - X - Y - Z
V
Vascularity (Cerebral)
Pronunciation: VAS-kyoo-LAIR-ih-tee
Definition: Cerebral vascularity refers to the density, architecture, and functional integrity of the circulatory network—comprising arteries, veins, and the micro-capillary bed—that services the central nervous system. It is the physical medium for Cerebral Blood Flow (CBF). Unlike peripheral vascularity, cerebral vascularity is governed by the Blood-Brain Barrier (BBB) and Neurovascular Coupling, a process where blood flow is dynamically shunted to specific brain regions in direct response to local neuronal activity. In nootropic research, optimizing vascularity is considered a "foundational" intervention, as neural performance is strictly limited by the rate of oxygen and glucose delivery.
The Nootropic Research Interface
Research into vascular nootropics (vasotropics) focuses on increasing the "bandwidth" of the brain’s life-support system.
- Neurovascular Coupling (NVC): This is the "on-demand" delivery system of the brain. When you focus on a task, your neurons signal the local blood vessels to dilate. Nootropics like Vinpocetine are researched for their ability to enhance this coupling, ensuring that the "fuel" arrives exactly where the "fire" of cognition is burning.
- Angiogenesis and VEGF: High-level research examines how certain compounds can stimulate Vascular Endothelial Growth Factor (VEGF) to grow new micro-capillaries. Increased capillary density reduces the diffusion distance between the blood and the mitochondria of the neuron, effectively "overclocking" the brain’s metabolic ceiling.
- Rheological Optimization: This refers to the "flow properties" of the blood itself. Some nootropics act as rheological agents by decreasing red blood cell aggregation (making blood less "viscous"). This allows blood to move through the narrowest capillaries of the prefrontal cortex with less resistance, improving oxygenation without increasing systemic blood pressure.
- Endothelial Health: The inner lining of brain blood vessels (the endothelium) produces Nitric Oxide (NO) to regulate vessel diameter. Research into Pycnogenol or Ginkgo Biloba often focuses on their ability to protect these endothelial cells from oxidative stress, preserving the brain's ability to self-regulate its blood flow.
Vascular Metrics in Cognitive Trials

Primary Research Methodologies
- fMRI (Functional MRI): Measures the BOLD (Blood-Oxygen-Level-Dependent) signal to map which areas of the brain are receiving increased vascular support during cognitive tasks.
- Transcranial Doppler (TCD): An ultrasound-based metric used to measure the velocity of blood flow through the major cerebral arteries in real-time.
- Near-Infrared Spectroscopy (NIRS): A non-invasive method used in nootropic trials to monitor oxygen saturation levels in the prefrontal cortex during intensive mental processing.
Research Note: A critical distinction must be made between Systemic Vasodilation and Cerebral-Selective Vasodilation. General vasodilators (like high-dose Niacin) can cause a "steal effect," where blood is diverted to the skin and muscles, actually reducing blood flow to the brain. Researchers prioritize "Cerebral-Selective" agents that specifically target the smooth muscle of the brain’s internal vasculature.
Vesicle
Pronunciation: VESS-ih-kul
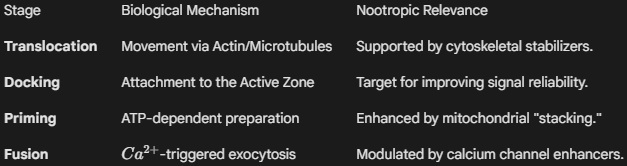
Definition: A synaptic vesicle is a spherical, lipid-bilayer organelle (approximately 35–50 nm in diameter) localized within the presynaptic bouton. Its primary function is to sequester neurotransmitters via active transport, protecting them from enzymatic degradation within the cytoplasm. Vesicles are the structural basis of Quantal Release—the theory that neurotransmitters are released in discrete, fixed amounts. The life cycle of a vesicle involves biogenesis, docking at the Active Zone, fusion-mediated exocytosis (triggered by Ca2+ influx), and subsequent endocytic recycling.
The Nootropic Research Interface
In nootropic science, the vesicle is viewed as the "ammunition" of the neuron. Enhancing vesicular efficiency ensures that the brain can maintain high-frequency signaling without "synaptic fatigue."
- Vesicular Filling and VMAT: The concentration of neurotransmitter within a vesicle is determined by transporters like VMAT2 (for monoamines) and VAChT (for acetylcholine). Nootropics that support the proton gradient (H+-ATPase) required for this filling ensure that each "quantum" released has maximum signaling potency.
- The Readily Releasable Pool (RRP): Not all vesicles are ready to fire. Researchers study the "RRP"—a small fraction of vesicles already docked at the membrane. Nootropics that increase the size or "priming" speed of the RRP, such as certain Racetams, are associated with faster reaction times and improved processing speed.
- SNARE Complex Assembly: For a vesicle to release its contents, it must "zip" together with the cell membrane using SNARE proteins. Research into Magnesium and Zinc levels often focuses on their roles as cofactors in this assembly, ensuring the "handshake" between the vesicle and the membrane is fluid and rapid.
- Vesicular Endocytosis: After release, the vesicle membrane must be reclaimed and remade. Nootropics that support Phospholipid synthesis (like Citicoline or Phosphatidylserine) provide the raw materials to repair and recycle vesicles, preventing the "crash" that occurs when a neuron runs out of storage packets during intense study or stress.
Vesicle Logistics in Nootropic Science
Primary Research Metrics
- Quantal Size (q): The physiological response (voltage change) elicited by the release of a single vesicle. An increase in q indicates that a nootropic has successfully increased the "payload" of each vesicle.
- FM 1-43 Imaging: A fluorescent dye technique used in in vitro research to visualize the rate of vesicle recycling and turnover in real-time.
- Vesicle-Associated Membrane Protein (VAMP) Levels: A biochemical marker used to quantify the total "storage capacity" of a neural tissue sample.
Research Note: Many "releasing agents" (like high-dose stimulants) can cause Vesicular Depletion, where vesicles are forced to release faster than they can be recycled. This leads to a profound "refractory period" or crash. Nootropic researchers prioritize Vesicular Potentiators—substances that make the release more efficient without emptying the entire reserve.
Vigilance Decrement
Pronunciation: VIH-jih-lunss DEH-kruh-ment
Definition: Vigilance decrement is the statistically significant decline in the rate of signal detection and the concurrent increase in reaction time that occurs during prolonged tasks requiring sustained attention. It is not merely a byproduct of "boredom," but a physiological phenomenon driven by the depletion of prefrontal catecholamines and the saturation of the brain's sensory processing buffers. In nootropic research, it is the primary metric used to evaluate the "durability" of a compound's cognitive effect, distinguishing between an acute stimulant "spike" and sustained cognitive "stamina."
The Nootropic Research Interface
Research into mitigating the decrement focuses on sustaining the "signal-to-noise" ratio in the brain during repetitive high-stakes monitoring.
- The Resource Depletion Model: This theory posits that vigilance requires a constant "draw" on metabolic resources. Nootropics that enhance Cerebral Glucose Utilization or Mitochondrial Efficiency (like ALCAR or Creatine) are studied for their ability to provide the ATP necessary to "power through" the 30-minute mark where the decrement typically begins.
- Arousal Regulation: According to the Over-arousal Hypothesis, the decrement can occur if a stimulant makes the subject too alert, leading to "attentional narrowing" and missed signals. Researchers use L-Theanine to modulate stimulants, aiming for a stable "flow state" that resists the decrement without inducing agitation.
- The Noradrenergic Bridge: Vigilance is governed by the Locus Coeruleus-Norepinephrine (LC-NE) system. Nootropics that act as selective Norepinephrine Reuptake Inhibitors (NRIs), such as Atomoxetine or certain adaptogens, are researched for their ability to maintain the "tonic" firing of the LC, which prevents the "attention gaps" characteristic of the decrement.
- Neural Noise Reduction: As the brain tires, "neural noise" increases, making it harder to distinguish a signal from the background. Researchers investigate Glutamatergic Modulators (like Racetams) to sharpen the "contrast" of sensory input, allowing the subject to maintain accuracy even as subjective fatigue increases.
The Anatomy of the Decrement

Primary Research Metrics
- Psychomotor Vigilance Task (PVT): The "gold standard" in sleep and nootropic research. It measures the frequency of "lapses" (response times >500 ms) during a 10-minute testing window.
- Sensitivity Index (d'): A Signal Detection Theory metric used to quantify a subject’s ability to discriminate between a signal and noise. A dropping d' value is a definitive marker of the decrement.
- Sustained Attention to Response Task (SART): Used to measure the "Go/No-Go" inhibitory control during the decrement, identifying if the subject begins responding impulsively to everything.
Research Note: When testing a new nootropic, it is vital to distinguish between Vigilance Decrement and Sleep Pressure. A compound like Caffeine might mask sleep pressure but still allow a vigilance decrement to occur. Conversely, eugeroics like Modafinil are unique in their ability to specifically target and flatten the vigilance decrement curve, maintaining high-fidelity signal detection for up to 12–16 hours.
